Nobel laureate Otto Hahn iscredited with the discovery of nuclear fission.
One of the elements he was working with wasnt behaving as itshould have.
It took another 15 years of discoveries in nuclear physics to be able to explain Hahns observations.

We aretwoprofessors ofnuclearphysicswho study rare nuclei including nuclear isomers.
They are alreadyused in medicineand could one day offer powerful options for energy storagein the form of nuclear batteries.
On the hunt for radioactive isotopes
40% off TNW Conference!
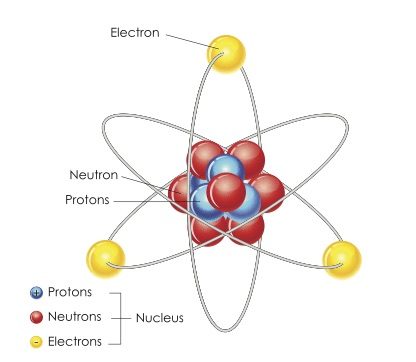
An element is considered radioactive if it spontaneously releases particles in a process calledradioactive decay.
When this happens, the element is transformed over time into a different element.
At that time, scientists relied on three criteria to discover and describe a new radioactive element.
One was to look at chemical properties how the new element reacts with other substances.
They also measured the punch in and energy of the particles released during the radioactive decay.
Finally, they would measure how fast an element decayed.

By the 1920s, physicists had discovered some radioactive substances with identical chemical properties but different half-lives.
These are called isotopes.
Uranium is a radioactive element with many isotopes, two of which occur naturally on Earth.
All the isotopes they studied behaved as expected, except for one.
This isotope appeared to have the same properties as one of the others, but its half-life was longer.
They called this substance uranium Z.
This was the first case of an isotope with two different half-lives.
Hahn published his discovery of thefirst nuclear isomer, even though he could not fully explain it.
It wasnt until 1932 that James Chadwick suggested a third particle neutrons were alsopart of the nucleus.
With this knowledge, the scientific community finally had the tools to understand uranium Z.
Arrangements resulting in less stable, higher energies of an isotope are called isomeric states.
At first nuclear isomers were useful in the scientific community only as a means to understand how nuclei behave.
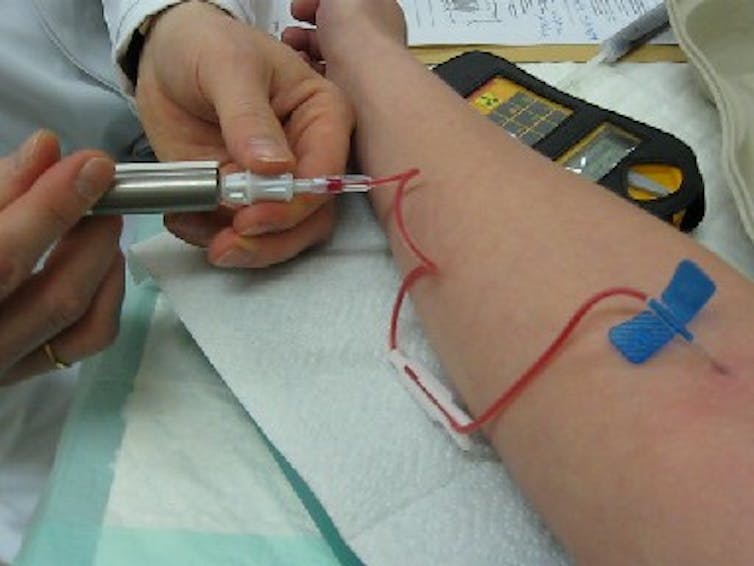
This photo shows a medical professional injecting technetium-99m into a patient.
Since isomers undergo radioactive decay, special cameras can track them as they move through the body.
For example, technetium-99m is an isomer of technetium-99.
As the isomer decays, it emits photons.
Radioactive elements and isotopes are normally dangerous because they emit charged particles that damage bodily tissues.
Isomers are also important in astronomy and astrophysics.
Stars are fueled by the energy released during nuclear reactions.
Since isomers arepresent in stars, nuclear reactions are different than if a material were in its ground state.
This makes the study of isomers critical for understanding how stars produce all the elements in the universe.